
R. Wayne Whitted MD, MPH
Paul A. Pietro MD
Marina Santana MMS, PA-C
Rebecca Karousatos MSRD, LD/N
8740 N Kendall Dr. Suite 101
Miami, Florida 33176
Phone: 305-596-3744
www.floridaamigos.com
Diagnosis and Treatment of Endometriosis
Endometriosis is a progressive disease affecting 5 to 10 percent of women. It can cause dyspareunia (pain with
intercourse), dysmenorrhea, low back pain, premenstrual spotting, and infertility. A definitive diagnosis can be
made only by means of laparoscopy, although MRI can often assist (if the radiologist’s training is adequate).
Medical treatment designed to interfere with ovulation generally provides effective pain relief, but the recurrence
rate following cessation of therapy is high, and this type of treatment will not resolve infertility. Minimally Invasive
Surgical treatment improves pregnancy rates (especially in the higher stages) and is the preferred initial treatment
for infertility caused by endometriosis. Surgery also appears to provide better long-term pain relief than medical
treatment. Bilateral oophorectomy and hysterectomy are treatment options for patients with intractable pain, if
childbearing is no longer desired. (Am Fam Physician 1999;60:1753-68.)
Endometriosis is characterized by the presence of endometrial tissue on the ovaries, fallopian tubes or other abnormal sites,
causing pain or infertility. The disease tends to progress under the repetitive influence of the menstrual cycle. Interrupting or
decreasing menstruation is the mainstay of medical therapy. The goal of surgery is to remove endometrial lesions.
Endometriosis is likely to remain problematic as long as menstruation persists. Fortunately, symptoms can be modulated or
alleviated with appropriate treatment.
Epidemiology
Women are usually 25 to 29 years old at the time of diagnosis, which is
frequently delayed in those who present with infertility rather than pain.
1
A
familial tendency has been identified.
2
Endometriosis has been found in 4.1
percent of asymptomatic women undergoing laparoscopy for sterilization;
however, evidence of the disease is present in 20 percent (range: 2 to 78
percent) of women undergoing laparoscopic investigation for infertility.
Approximately 24 percent (range: 4 to 82 percent) of women who complain
of pelvic pain are subsequently found to have endometriosis.
3
The overall
prevalence, including symptomatic and asymptomatic women, is estimated
to be 5 to 10 percent.
4
Because surgical confirmation is necessary for the
diagnosis, the true prevalence of the disease is unknown.
Endometriosis should be
considered in women who develop
dysmenorrhea after years of pain-
free cycles.
The Association of Minimally Invasive Gynecologic Surgeons
…dedicated to safe, state-of-the-art surgery and health life-styles for women of all ages
Pathogenesis
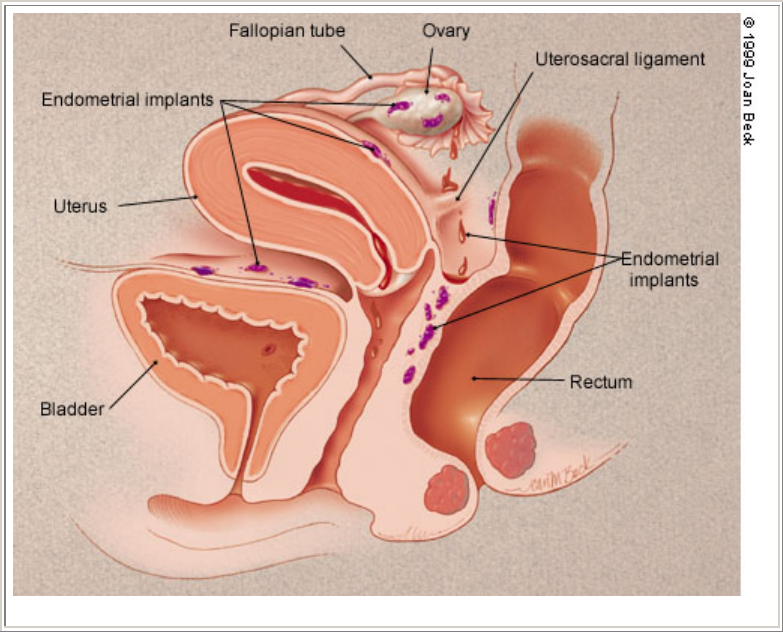
Endometriosis is not well understood and is probably multi-factorial in origin. The most widely embraced theory involves
retrograde menstruation
5
(Figure 1). In endometriosis the refluxed cells may implant in the pelvis, bleed in response to cyclic
hormonal stimulation and increase in size along with progression of symptoms.
6
Immune alterations may also contribute to the
persistence of implants or endometriosis-associated infertility.
7,8
Two other theories have received support. One holds that peritoneal epithelium can be "transformed" into endometrial tissue,
perhaps because of chronic inflammation or chemical irritation from refluxed menstrual blood. This theory of "coelomic
metaplasia" is based on the observation that coelomic epithelium is the common ancestor of endometrial and peritoneal cells,
thus allowing transformation of one type of cell into another. A final theory hypothesizes that müllerian remnants can
differentiate into endometrial tissue. The circumstances in which this would occur are not clear but, once endometrium is
present, it will cause symptoms in a cyclic fashion.
Although retrograde menstruation seems almost certain to be involved in the pathogenesis of endometriosis, that theory does
not explain the full spectrum of the disease. For example, endometrial implants are occasionally found in such remote sites as
the lung or even the nose. Moreover, endometriosis also occurs, albeit rarely, in men taking large doses of estrogen. The
theories of coelomic metaplasia and müllerian remnant differentiation are better suited than the theory of retrograde
menstruation to explain some of these exceptional circumstances.
FIGURE 1. Basic anatomy of retrograde menstruation.
Clinical Features and Diagnostic Evaluation
Endometriosis should be considered in any woman of reproductive age who has pelvic pain (Table 1). The most common
symptoms are dysmenorrhea, dyspareunia and low back pain that worsens during menses.
9
Depending on the location of the
implants, rectal pain and painful defecation may also occur. The diagnosis of endometriosis should be considered especially if
a patient develops dysmenorrhea after years of pain-free menstrual cycles. Of course, other causes of secondary dysmenorrhea
and chronic
pelvic pain (e.g., upper genital tract infections, adenomyosis, and adhesions) may produce
similar symptoms.

Infertility may also be the presenting complaint. Infertile
patients often have no painful symptoms, and their disease is
only uncovered in the course of the diagnostic work-up for
infertility. The reason for this divergence in clinical
manifestations is unknown.
Physical examination should be performed during early
menses, when implants are likely to be largest and most
tender. The physician should palpate for a fixed, retroverted
uterus, adnexal and uterine tenderness, pelvic masses or
nodularity along the uterosacral ligaments. A rectovaginal
examination is required to identify uterosacral, cul-de-sac or
septal nodules. However, most women with endometriosis
have normal pelvic findings, and laparoscopy is necessary
for definitive diagnosis. Although no single laboratory test
has shown reliable clinical utility, it is possible that
eventually a combination of biochemical markers and
clinical assessment will decrease the need for surgical
confirmation.
10,11
Pelvic ultrasonography, computed tomography and magnetic
resonance imaging are occasionally used to identify
individual lesions, but these modalities are not helpful in
assessing the extent of endometriosis.
12
Even with direct
visualization, diagnosis of endometriosis can be difficult.
Lesions appear in multiple guises that are at times difficult to
interpret. This diagnostic challenge is compounded by the
unreliable correlation between clinical manifestations and
surgical findings.
13
A patient who is asymptomatic or has
very mild symptoms may have extensive disease, whereas an
infertile patient may have very few implants. A better
correlation between clinical and surgical disease may be
observed in more severe cases: in at least one study
14
it has
been found that women with severe, chronic pelvic pain
have a more advanced stage of disease at initial diagnosis.
The American Fertility Society's revised staging instrument
can help standardize findings and document the patient's
baseline condition and subsequent progress.
15
Staging is
based on location, diameter and depth of lesions, and density
of adhesions. Stages range from minimal to severe disease.
Despite this standardization, the correlation between stage
and extent of disease remains controversial.
Treatment
In most patients, confirmatory laparoscopy is required before
treatment is instituted.
4
In women with few symptoms, an
empiric trial of oral contraceptives or progestins may be
warranted to assess pain relief. Recently, an empiric three-
month trial of therapy with gonadotropin-releasing hormone
(GnRH) analogs has been a popular strategy.
16
In severe or
unresponsive cases, or in the investigation of infertility,
exact diagnosis is required to direct management and to justify possibly
unpleasant medical treatments. Patients with
infertility should undergo a thorough basic evaluation for other causes of infertility before diagnostic
laparoscopy is undertaken.
TABLE 1
Differential Diagnosis of Endometriosis
by Symptom
Generalized pelvic pain
Pelvic
inflammatory
disease
Endometritis
Pelvic adhesions
Neoplasms,
benign or
malignant
Ovarian torsion
Sexual or
physical abuse
Nongynecologic
causes
Dysmenorrhea
Primary
Secondary
(adenomyosis,
myomas,
infection,
cervical
stenosis)
Dyspareunia
Musculoskeletal
causes (pelvic
relaxation,
levator spasm)
Gastrointestinal
tract
(constipation,
irritable bowel
syndrome)
Urinary tract
(urethral
syndrome,
interstitial
cystitis)
Infection
Pelvic vascular
congestion
Diminished
lubrication or
vaginal
expansion
because of
insufficient
arousal
Infertility
Male factor
Tubal disease
(infection)
Anovulation
Cervical factors
(mucus, sperm
antibodies,
stenosis)
Luteal phase
deficiency
Adapted with permission from Ryder RM. Chronic pelvic
pain. Am Fam Physician 1996;54:2225.

Treatment may be expectant, or a patient may choose either medical or surgical options. Infertile patients may increase the
likelihood of subsequent conception by undergoing surgery, but medical treatment has not been shown to help these patients
conceive.
17,18
Furthermore, pregnancy is contraindicated in patients receiving medical treatment and is in fact unlikely, because
the drugs that are used interfere with ovulation. Medical and surgical approaches have been successful in reducing the pain
associated with endometriosis.
Medical Treatment
Medical treatment should be reserved for use in patients with pain or dyspareunia, because no pharmacologic method appears
to restore fertility.
Danazol. Danazol (Danocrine) has been highly effective in relieving the
symptoms of endometriosis, but adverse effects may preclude its use.
(There are now other treatments that may be better tolerated.) Danazol is a
synthetic androgen that inhibits leuteinizing hormone (LH) and follicle-
stimulating hormone (FSH), resulting in a relatively hypoestrogenic state.
Endometrial atrophy is the likely mechanism in the relief of pain from
endometriosis. Adverse effects related to estrogen defiency include
headache, flushing, sweating and atrophic vaginitis. Androgenic side effects
include acne, edema, hirsutism, deepening of the voice and weight gain.
Danazol therapy should be started when the patient is menstruating. The initial dosage should be 800 mg per day, given in two
divided oral doses, but this dosage can be titrated down as long as amenorrhea persists and pain symptoms are controlled.
Patients with less severe symptoms may be given 200 to 400 mg per day, in two divided oral doses. Treatment duration is six
months but can be extended to nine months in responsive patients with severe disease. The overall response rate is 84 to 92
percent, with beneficial effects lasting up to six months after treatment has stopped.
12
TABLE 2
Medical Treatment of Endometriosis
Drug
Dosage Adverse effects
Cost*
Danazol (Danocrine) 800 mg per day in 2 divided doses Estrogen deficiency,
androgenic side
effects
$410,
brand
367,
generic
Oral contraceptives 1 pill per day (continuous or cyclic) Headache, nausea,
hypertension
29
brand†
24 to
27‡,
generic
Medroxyprogesterone
suspension (Depo-Provera)
100 mg IM every 2 weeks for 2
months; then 200 mg IM every
month for 4 months or 150 mg IM
every 3 months
Weight gain,
depression, irregular
menses or
amenorrhea
22, brand
13,
generic
Medroxyprogesterone
(Provera)
5 to 20 mg orally per day Same as with other
oral progestins
Norethindrone acetate
(Aygestin)
5 mg per day orally for 2 weeks;
then increase by 2.5 mg per day
every 2 weeks up to 15 mg per day
Same as with other
oral progestins
113§
Because no pharmacologic method
appears to restore fertility, medical
treatment for endometriosis should be
reserved for use in patients with pain or
dyspareunia.

Leuprolide (Lupron) 3.75 mg IM every month for 6
months
Decrease in bone
density, estrogen
deficiency
371,
brand
318,
generic
Gosarelin (Zoladex) 3.6 mg SC (in upper abdominal
wall) every 28 days
Estrogen deficiency 470
Nafarelin (Synarel) 431 400 mg per day: 1 spray in 1 nostril
in a.m.; 1 spray in other nostril in
p.m.; start treatment on day 2 to 4
of menstrual cycle
Estrogen deficiency,
bone density changes,
nasal irritation
IM = intramuscularly; SC = subcutaneously.
*--Estimated cost to the pharmacist based on average wholesale prices (rounded to the nearest dollar) for one
month of treatment at the lowest dosage level in Red book. Montvale, N.J.: Medical Economics Data, 1999.
Cost to patient will be greater, depending on prescription filling fee.
†--Cost based on prices of Lo-Ovral 28 and Ortho-Novum.
‡--Cost based on generic versions of Lo-Ovral 28 and Ortho-Novum.
§--For one month's therapy at 15 mg per day.
GnRH Agonists. These agents (e.g., leuprolide [Lupron], gosarelin [Zoladex]) inhibit the secretion of gonadotropin and are
comparable to danazol in relieving pain.
12-19
Like danazol, GnRH agonists are contraindicated in pregnancy and have
hypoestrogenic side effects. In particular, they have been shown to produce a mild degree of bone loss, although this condition
reverses after the medication is discontinued. Because of concerns about osteopenia, "add-back" therapy with low-dose
estrogen has been recommended but is not currently an FDA-labeled indication for estrogen replacement therapy.
20,21
The dosage of leuprolide is a single monthly 3.75-mg depot injection given intramuscularly. Gosarelin, in a dosage of 3.6 mg,
is administered subcutaneously every 28 days. A nasal spray (nafarelin [Synarel]) is also available and is used twice daily. The
response rate is similar to that with danazol; about 90 percent of patients experience pain relief. The pregnancy rate after the
use of these agents is no different from that in untreated patients.
Oral Contraceptive Pills. Oral contraceptive pills (OCPs) suppress LH and FSH and prevent ovulation. They also have direct
effects on endometrial tissue, rendering it thin and compact. The decidualization of endometrial implants, coupled with reduced
reflux related to lower menstrual volume, is the probable mechanism of pain relief with OCPs, making them comparable to
other treatments in effect.
9
Combination OCPs alleviate symptoms in about three quarters of patients. No hormonal
combination appears to be more effective than another. They can be taken continuously (with no placebos) or cyclically, with a
week of placebo pills between cycles. The OCPs can be discontinued after six to 12 months or continued indefinitely,
depending on such factors as patient satisfaction and the desirability of pregnancy.
Progestational Agents. Progestins are similar to combination OCPs
in their effects on FSH, LH and endometrial tissue. They may be
associated with more bothersome adverse effects than OCPs and, if a
depot form (i.e., medroxyprogesterone suspension [Depo-Provera]) is
used, return to fertility may be delayed. Nonetheless, progestins are
effective in reducing the symptoms of endometriosis. One study that
pooled data from 14 investigations found no significant difference
between the efficacy of progestins and that of any other medical
treatment.
22
Although this conclusion was based on analysis of the
combined results of a handful of small, heterogeneous studies, it is
important because progestins are much cheaper than either danazol or
GnRH analogs.
TABLE 3
Surgical vs. Medical Treatment of
Endometriosis
Advantages Disadvantages Treatment
Given the likelihood of comparable efficacy, as well as the certainty
of a high rate of recurrence regardless of the agent used, physicians
may elect to prescribe OCPs or progestins as first-line agents on the
basis of cost alone. If effective, these agents can be used safely for
long periods of time. Progestins can be given orally on a daily basis
or delivered by injection. Oral regimens may include once-daily
administration of medroxyprogesterone at the lowest effective dosage
(5 to 20 mg). Depot medroxyprogesterone has been given
intramuscularly every two weeks for two months at 100 mg per dose
and then once a month for four months at 200 mg per dose. Medical
treatments are reviewed in Table 2.
Surgical Treatment
Surgical treatment is the preferred approach to infertile patients with
advanced endometriosis.
12
The benefit of surgery in these patients
may be due entirely to the mechanical clearance of adhesions and
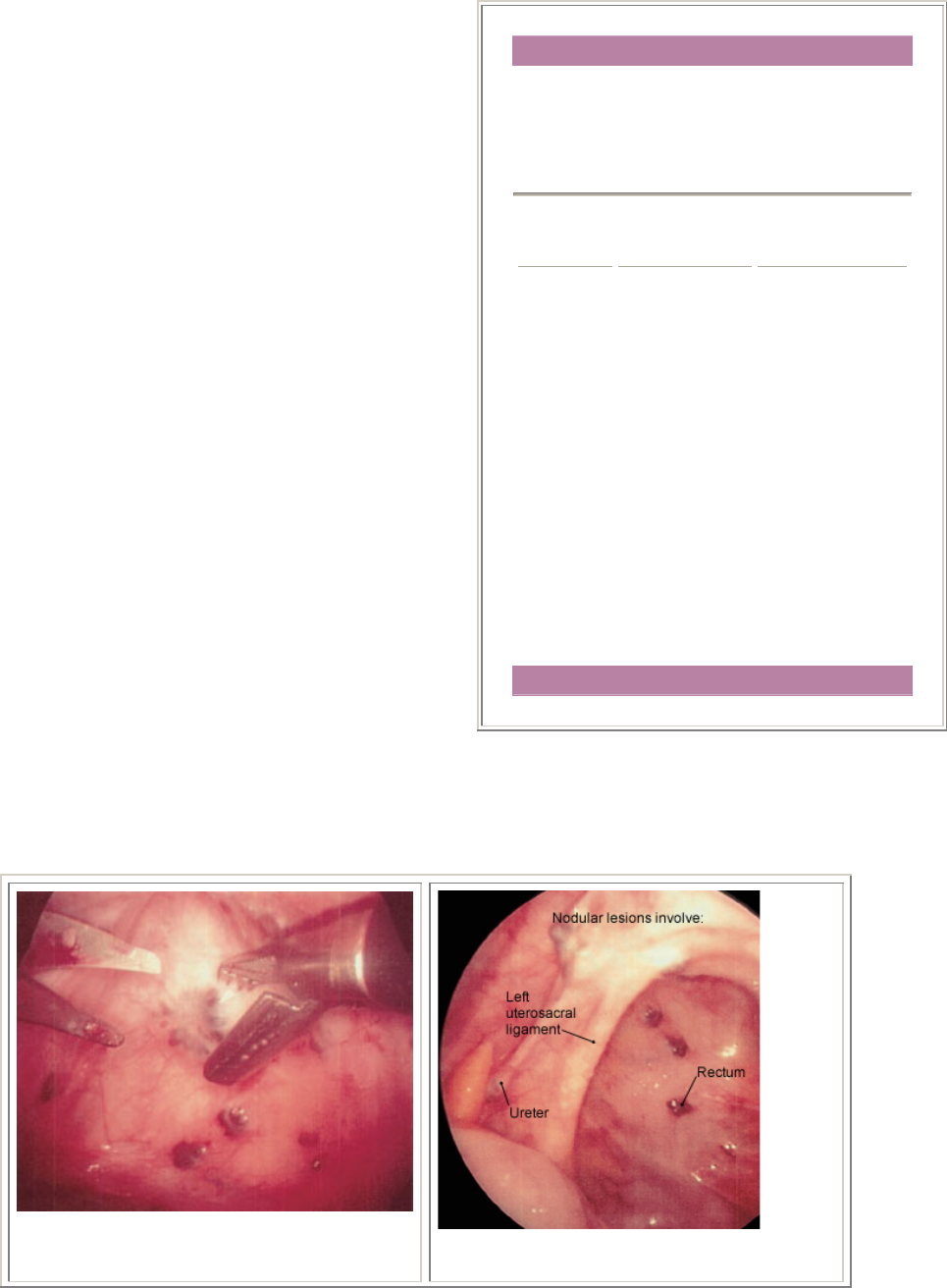
obstructive lesions (Figure 2). Some of the endometrial lesions are
cystic or nodular and can be excised (Figures 3, 4 and 5), while some
are hemorrhagic or petechial and amenable to laser obliteration
(Figures 6 and 7). Until recently, surgery in infertile patients with limited disease was thought to be no better than expectant
management. However, a recent randomized, controlled study involving 341 infertile women with minimal or mild
endometriosis demonstrated a 13 percent absolute increase in the probability of pregnancy in a 36-week period.
23
Infertile
patients with documented endometriosis can benefit from the same reproductive techniques (e.g., superovulation, in vitro
fertilization) that are used in other infertile patients.
24,25
FIGURE 2. Laparoscopic excision of nodular
endometrial lesions ov rlying the rectum.
e
FIGURE 4. Nodular endometrial lesions in
the posterior cul-de-sac.
Surgical Beneficial for
infertility
Possibly better
long-term
results
Definitive
diagnosis
Option for
definitive
treatment
Expensive
Invasive
Medical Decreased
initial cost
Empiric
treatment
Effective for
pain relief
Adverse effects
common
Unlikely to
improve fertility
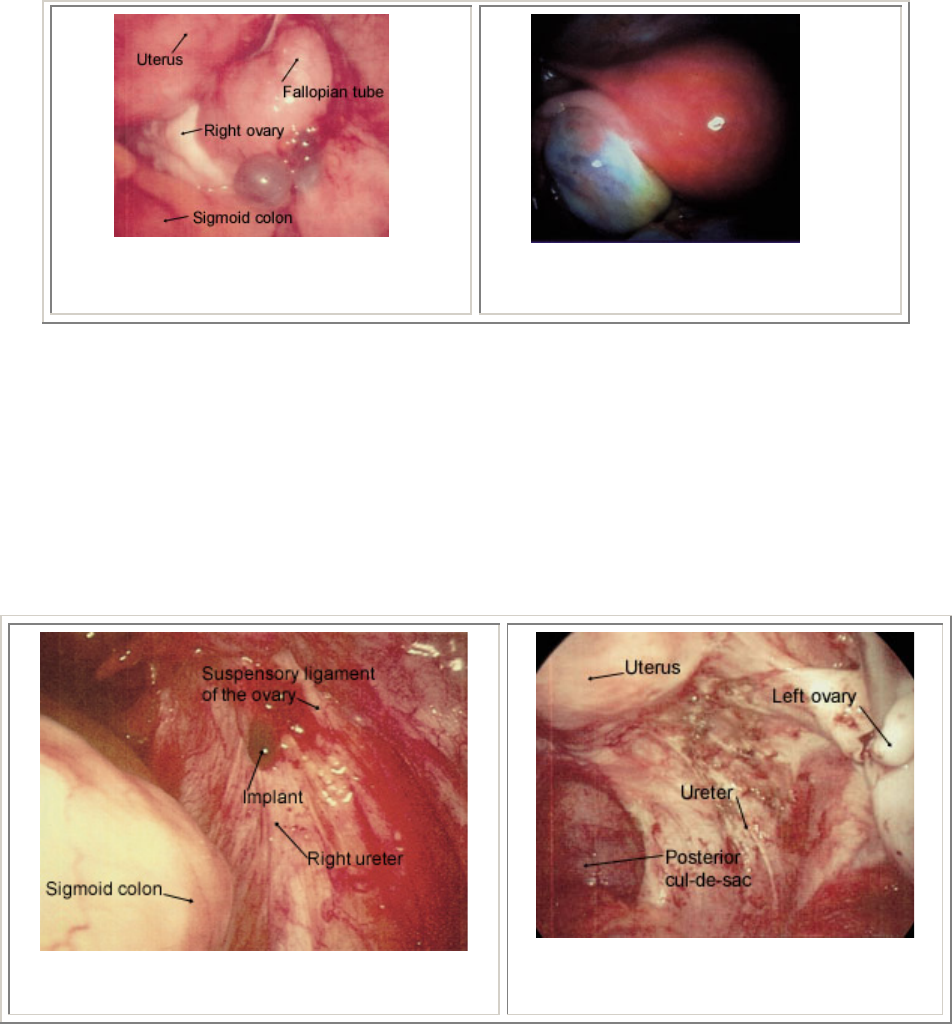
FIGURE 3. Cystic implants
adjacent to the right ovary; note
bluish appearance.
FIGURE 5. Ovary with
endometrioma.
The usefulness of conservative surgery for pain relief is unclear, but it appears that immediate postoperative efficacy is at least
as high as with medical treatment, and long-term outcomes may be considerably higher.
26
Laparoscopy is much more
expensive than medical treatment, however, causing some physicians to argue that overall costs can be reduced by aggressive
use of empiric treatments before surgery is considered.
16
Table 3 summarizes the advantages and disadvantages of medical and
surgical treatments.
Definitive surgery, which includes hysterectomy and oophorectomy, is reserved for use in women with intractable pain who no
longer desire pregnancy.
27
In less severe cases, one ovary may be retained to preserve ovarian function, although improvement
will be less definitive. Women who have undergone oophorectomy should be treated with estrogen replacement, even at the
risk of some recurrence.
27
FIGURE 7. Extensive endometriosis in the
ovarian fossa. Lesions have a petechial
appearance.
FIGURE 6. Hemorrhagic lesions overlying the
right ureter.
In practical terms, when the diagnosis of endometriosis is made at laparoscopy, surgical ablation of lesions is frequently
performed. Thus, because laparoscopic diagnosis is usually recommended before instituting treatment, most women with
endometriosis undergo surgical therapy initially. It is generally agreed that an expert surgeon who is extensively trained in
ablation procedures will have the best outcomes
9
(Figure 8).
Recurrence Rates
Perhaps the strongest reason for beginning with surgical treatment is the apparently lower recurrence rate compared with
medical treatment.
27
Early studies of conservative surgical therapy showed a laparoscopically defined cumulative five-year
recurrence rate of about 19 percent.
28,29
The long-term benefit of surgical intervention for pain is enhanced by definitive
surgery, including bilateral oophorectomy, with a 10 percent cumulative recurrence after 10 years.
27
This rate is considerably
lower than those following medical therapy. In one study of recurrence after medical treatment, cumulative five-year rates of
recurrence were 53.4 percent.
30
Unfortunately, patients whose presenting complaint was pain and those seeking treatment for
infertility were grouped together in the analysis. Other studies show similar recurrence rates, regardless of the medical therapy
used.
26
At least one study noted higher recurrence rates in patients with more advanced stages of disease.
30
Combining or repeating treatments may result in better long-term outcomes, but studies of combined treatments are
inconclusive because of lack of randomization, small sample size or insufficient follow-up time. One randomized, double-blind
study
31
showed additional pain relief and objective improvement with immediate postoperative treatment with danazol or
medroxyprogesterone, but the study ended with a second laparoscopy after six months, too soon to identify longer-term
benefits. In a more recent investigation, it was found that the best and only statistically significant long-term outcomes were
achieved with surgery followed by danazol treatment; however, the study was limited by a small sample size.
32
Although few
studies have been conducted to evaluate retreatment with danazol or GnRH analogs, repeated administrations of these drugs are
theoretically an option and are probably safe at appropriate intervals.
33
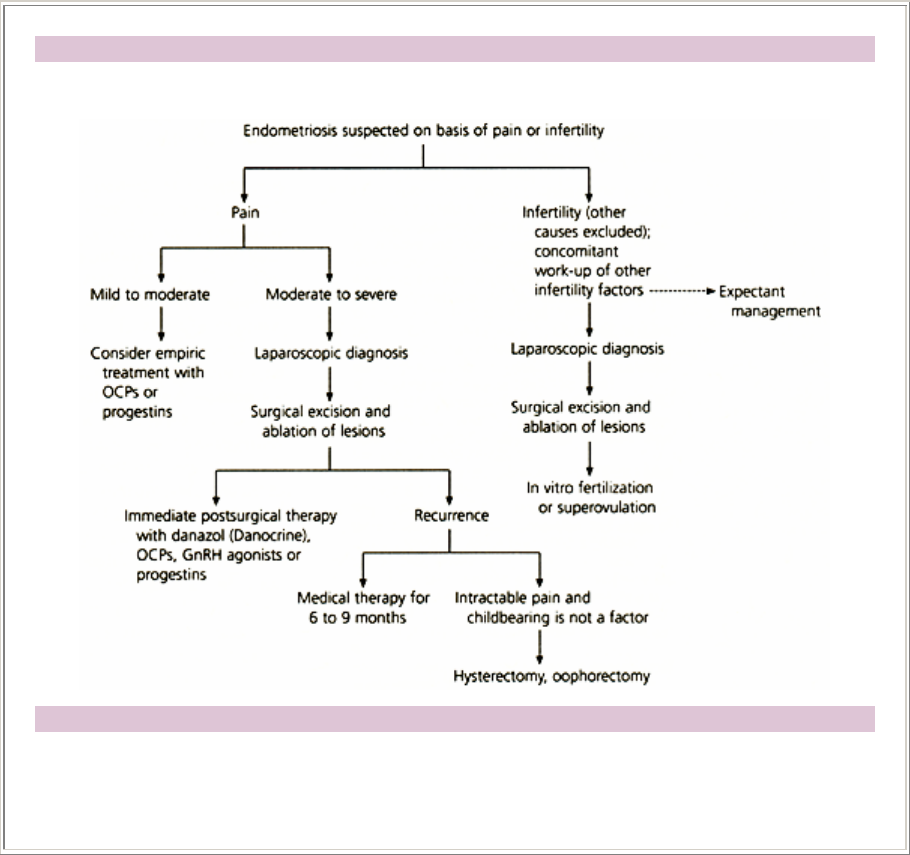
Algorithm for treating endometriosis
Treatment of Endometriosis
FIGURE 9.Algorithm for treating endometriosis based on presenting symptom of either pain or
infertility. (Broken arrow = optional consideration; OCPs = oral contraceptive pills; GnRH =
gonadotropin-releasing hormone)
The Author
CAROLINE WELLBERY, M.D.,
is an assistant professor in the Department of Family Medicine at Georgetown University School of
Medicine, Washington, D.C. Dr. Wellbery graduated from the University of California, San Francisco,
School of Medicine, and completed a residency in family practice at the Santa Rosa (Calif.) Community
Hospital. She serves as assistant deputy editor for American Family Physician.
Address correspondence to Caroline Wellbery, M.D., Department of Family Medicine, Georgetown University
Medical Center, 3800 Reservoir Rd., Washington, DC 20007. Reprints are not available from the author.
REFERENCES
1. Dmowski WP, Lesniewicz R, Rana N, Pepping P, Noursalehi M. Changing trends in the diagnosis of endometriosis: a
comparative study of women with pelvic endometriosis presenting with chronic pelvic pain or infertility. Fertil Steril
1997;67:238-43.
2. Moen MH, Magnus P. The familial risk of endometriosis. Acta Obstet Gynecol Scand 1993;72: 560-4.
3. Eskenazi B, Warner M. Epidemiology of endometriosis. Obstet Gynecol Clin North Am 1997; 24:235-58.
4. Lu PY, Ory SJ. Endometriosis: current management. Mayo Clin Proc 1995;70:453-63.
5. Thomas EJ. Endometriosis, 1995--confusion or sense? Int J Gynecol Obstet 1995;48:149-55.
6. Brosens IA. Endometriosis--a disease because it is characterized by bleeding. Am J Obstet Gynecol 1997;176:263-7.
7. Gleicher N. Immune dysfunction--a potential target for treatment in endometriosis. Br J Obstet Gynaecol 1993;102(12
suppl):4-7.
8. Martinez-Roman S, Balasch J, Creus M, Fabregues F, Carmona F, Vilella R, et al. Immunological factors in
endometriosis-associated reproductive failure: studies in fertile and infertile women with and without endometriosis.
Hum Reprod 1997;12:1794-9.
9. American College of Obstetricians and Gynecologists. Endometriosis. ACOG technical bulletin no. 184. Washington,
D.C.: ACOG, 1993.
10. Medl M, Ogris E, Peters-Engl C, Mierau M, Buxbam P, Leodolter S. Serum levels of the tumour-associated trypsin
inhibitor in patients with endometriosis. Br J Obstet Gynaecol 1997;104:78-81.
11. Brinton DA, Quatrociocchi-Longe TM, Kiechle FL. Endometriosis: identification by carbonic anhydrase
autoantibodies and clinical features. Ann Clin Lab Sci 1996;26:409-20.
12. Olive D, Schwartz LB. Endometriosis. N Engl J Med 1993;328:1759-69.
13.
Ripps BA, Martin DC. Correlation of focal pelvic tenderness with implant dimension and stage of endometriosis. J
Reprod Med 1992:37:620-4.
14. Stovall DW, Bowser LM, Archer DF, Guzick DS. Endometriosis-associated pelvic pain: evidence for an association
between the stage of disease and a history of chronic pelvic pain. Fertil Steril 1997; 68:13-8 [Published erratum in
Fertil Steril 1998;69: 979].
15.
Revised American Fertility Society classification of endometriosis. Fertil Steril 1985;43:351-2.
16.
Heinrichs WL, Henzl MR. Human issues and medical economics of endometriosis. J Reprod Med 1998;43(3
suppl):299-308.
17.
Hull ME, Moghissi KS, Magyar DF, Hayes MF. Comparison of different treatment modalities of
endometriosis in infertile women. Fertil Steril 1987; 47:40-4.
18. Telimaa S, Puolakka J, Ronnberg L, Kauppila A. Placebo-controlled comparison of danazol and high-dose
medroxyprogesterone acetate in the treatment of endometriosis. Gynecol Endocrinol 1987;1:13-23.
19.
Bromham DR, Booker MW, Rose GL, Wardle PG, Newton JR. Updating the clinical experience in endometriosis--the
European perspective. Br J Obstet Gynaecol 1995;102(12 suppl):12-6.
20. Kiesel L, Schweppe KW, Sillem M, Siebzehnrubl E. Should add-back therapy for endometriosis be deferred for
optimal results? Br J Obstet Gynaecol 1996;103(14 suppl):15-7.
21. Moghissi KS. Add-back therapy in the treatment of endometriosis: the North American experience. Br J Obstet
Gynaecol 1996;103(14 suppl):14.
22.
Vercellini P, Cortesi I, Crisgnani PG. Progestins for symptomatic endometriosis: a critical analysis of the evidence.
Fertil Steril 1997;68:393-401.

23. Marcoux S, Maheux R, Berube S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. N
Engl J Med 1997;337:217-22.
24. Tummon IS, Asher LJ, Martin JS, Tulandi T. Randomized controlled trial of superovulation and insemination for
infertility associated with minimal or mild endometriosis. Fertil Steril 1997;68: 8-12.
25. Kodama H, Fukuda J, Karube H, Matsui T, Shimizu Y, Tanaka T. Benefit of in vitro fertilization treatment for
endometriosis-associated infertility. Fertil Steril 1996;66:974-9.
26. Revelli A, Modotti M, Ansaldi C, Massobrio M. Recurrent endometriosis: a review of biological and clinical aspects.
Obstet Gynecol Surv 1995;50:747-54.
27. Namnoum AB, Hickman TN, Goodman SB, Gehlbach DL, Rock JA. Incidence of symptom recurrence after
hysterectomy for endometriosis. Fertil Steril 1995;64:898-902.
28. Redwine DB. Conservative laparoscopic excision of endometriosis by sharp dissection: life table analysis of
reoperation and persistent or recurrent disease. Fertil Steril 1991;56:628-34.
29. Wheeler JM, Malinak LR. Recurrent endometriosis. Contrib Gynecol Obstet 1987;16:13-21.
30. Waller KG, Shaw RW. Gonadotropin-releasing hormone analogues for the treatment of endometriosis: long-term
follow-up. Fertil Steril 1993;59:511-5.
31. Telimaa S, Ronnberg L, Kauppila A. Placebo-controlled comparison of danazol and high-dose medroxyprogesterone
acetate in the treatment of endometriosis after conservative surgery. Gynecol Endocrinol 1987;1:363-71.
32. Mahmood TA, Templeton A. The impact of treatment on the natural history of endometriosis. Hum Reprod
1990;5:965-70.
33. Hornstein MD, Yuzpe AA, Burry K, Buttram VC Jr, Heinrichs LR, Soderstrom RM, et al. Retreatment with nafarelin
for recurrent endometriosis symptoms: efficacy, safety, and bone mineral density. Fertil Steril 1997;67:1013-8.
Copyright © 1999 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that
printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied,
printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in
writing by the AAFP. Contact
afpserv@aafp.org for copyright questions and/or permission requests.